Terpene synthases in disguise: enzymology, structure, and opportunities of non-canonical terpene synthases
Abstract
Covering: up to July 2019
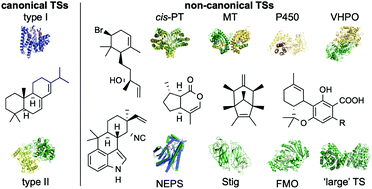
Terpene synthases (TSs) are responsible for generating much of the structural diversity found in the superfamily of terpenoid natural products. These elegant enzymes mediate complex carbocation-based cyclization and rearrangement cascades with a variety of electron-rich linear and cyclic substrates. For decades, two main classes of TSs, divided by how they generate the reaction-triggering initial carbocation, have dominated the field of terpene enzymology. Recently, several novel and unconventional TSs that perform TS-like reactions but do not resemble canonical TSs in sequence or structure have been discovered. In this review, we identify 12 families of non-canonical TSs and examine their sequences, structures, functions, and proposed mechanisms. Nature provides a wide diversity of enzymes, including prenyltransferases, methyltransferases, P450s, and NAD+-dependent dehydrogenases, as well as completely new enzymes, that utilize distinctive reaction mechanisms for TS chemistry. These unique non-canonical TSs provide immense opportunities to understand how nature evolved different tools for terpene biosynthesis by structural and mechanistic characterization while affording new probes for the discovery of novel terpenoid natural products and gene clusters via genome mining. With every new discovery, the dualistic paradigm of TSs is contradicted and the field of terpene chemistry and enzymology continues to expand.


 Please wait while we load your content...
Please wait while we load your content...