D–A type luminophores with a twisted molecular conformation constructed by phenoxazine and diphenylsulfone showing high contrast mechanofluorochromism†
Abstract
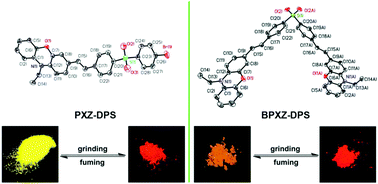
Two D–A type luminophores PXZ-DPS and BPXZ-DPS exhibiting a twisted molecular conformation are synthesized and characterized. Owing to the strong intramolecular charge-transfer (ICT) effect, both the compounds exhibit intense emissions in nonpolar and medium polarity solvents but in highly polar solvents their fluorescence is very weak or nearly completely quenched. Moreover, they show high-contrast morphology-dependent mechanofluorochromism. Upon grinding, colour changes from an initial bright yellow-green and orange-yellow to final orange-red, respectively, are observed. Meanwhile, the emission wavelengths of PXZ-DPS and BPXZ-DPS powders red shift from 548 nm and 569 nm to 601 nm and 609 nm, respectively, indicating large spectral red-shifts of 53 nm and 40 nm. PXRD and spectral data show that the reversible mechanofluorochromic (MFC) behavior of PTZ-DPS and BPTZ-DPS comes from the phase transition between the crystalline and amorphous states, and the planarization of the molecular conformation and subsequent planar intramolecular charge transfer (PICT) upon grinding are responsible for the red-shift in the PL spectrum. Based on the results of X-ray single-crystal analysis, it is found that the twisted rigid structure and multiple intermolecular hydrogen-bonds in the crystals of PTZ-DPS and BPXZ-DPS contribute to their effective solid-state fluorescence and obvious MFC behavior. In addition, it is worth noting that PXZ-DPS exhibit higher solid-state luminescence efficiency and a more significant MFC behavior than those of BPXZ-DPS. The reason is as follows: for BPXZ-DPS, there are π–π interactions between molecules besides the multiple hydrogen-bonding interactions in packing diagrams. However, no π–π interactions in crystals are present for PXZ-DPS. It means that in the crystals PXZ-DPS possesses a looser and more easily destructible molecular packing than that of BPXZ-DPS, which is beneficial for solid-state fluorescence and the observation of the MFC phenomenon.


 Please wait while we load your content...
Please wait while we load your content...