Insights into water permeability and Hg2+ removal using two-dimensional nanoporous boron nitride†
Abstract
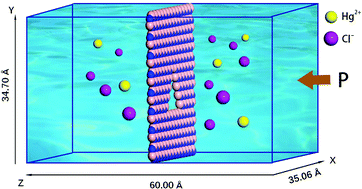
Industrial wastewater containing Hg2+, when discharged into nature, will pose a serious threat to ecological security. In order to separate Hg2+ from sewage, we investigate the potential applications of hexagonal boron nitride (h-BN) for Hg2+ removal using molecular dynamics simulations. The relationships between water flux and pore size, pore chemistry and applied pressure are analyzed, and the filtration mechanism of Hg2+ is also discussed in detail. The calculated thermodynamic data are compared with the measured values in the related literature. The results indicate that water flux is proportional to pore size and applied pressure. The N-edged pore can induce water molecules to pass through the nanopore. The potential barrier and the hydration are beneficial to Hg2+ rejection. When the pore diameter is smaller than the second hydration radius of Hg2+, the efficiency of Hg2+ removal is high. In particular, the Hg2+ rejection ratio of B5 is 100%, and water permeability is also 2–3 orders of magnitude higher than that of traditional reverse osmosis membranes. Overall, this work plays a guiding role in the liquid phase separation of heavy metal ions.


 Please wait while we load your content...
Please wait while we load your content...