Formation of iron(iii)–thiolate metallocyclophane using a ferrocene-based bis-isocyanide†
Abstract
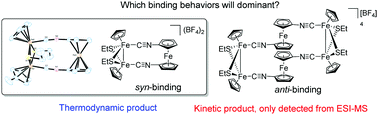
Two ferrocene–isocyanide ligands, 1-isocyanoferrocene (FcNC) and 1,1′-diisocyanoferrocene [Fc(NC)2], were observed to react with the redox active iron–thiolate core complexes [(CpFe)2(μ-SEt)2(CH3CN)2][BF4]2 (1[BF4]2) to form the respective ferrocenyl–isocyanide adducts, [(CpFe)2(μ-SEt)2(FcNC)2][BF4]2 (2[BF4]2) and [(CpFe)2(μ-SEt)2(Fc(NC)2)][BF4]2 (3[BF4]2). By using ESI-MS, we demonstrated that the kinetic product in the route for the formation of 3[BF4]2 from 1[BF4]2 and Fc(NC)2 is complex [(CpFe)2(μ-SEt)2(Fc(NC)2)2][BF4]2 (4[BF4]2). The kinetic product, 4[BF4]2, would gradually transform to its corresponding thermodynamic product (3[BF4]2) due to the syn-orientation effect of Fc(NC)2 and syn ligand directed binding site of the iron–thiolate core system. The results of structural, spectroscopic and electrochemical characterization of new iron–thiolate core complexes are described. Chemical reduction and oxidation reactions of 2[BF4]2 and 3[BF4]2 were examined to elucidate the nature of the species generated in the electrochemical measurement.


 Please wait while we load your content...
Please wait while we load your content...