Synthesis and comparative studies of K-region functionalized pyrene derivatives†
Abstract
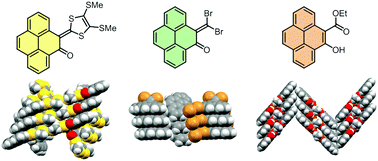
Two new K-region functionalized pyrene derivatives, namely 5-(dibromomethylene)pyren-4(5H)-one and 5-(1,3-dithiol-2-ylidene)pyren-4(5H)-one, were synthesized through olefination reactions of pyrene-4,5-dione. In addition, another pyrene derivative, ethyl 5-hydroxypyrene-4-carboxylate, was acquired as a byproduct in the synthesis. Electronic, electrochemical redox, and molecular structural properties of these pyrene derivatives were characterized by UV-Vis absorption, cyclic voltammetric, and X-ray single crystallographic analyses in combination with density functional theory (DFT) calculations. The roles of various intramolecular and intermolecular noncovalent forces in molecular conformation and crystal packing were investigated through various theoretical calculations, including natural bond orbital (NBO), quantum theory of atoms in molecules (QTAIM), noncovalent interaction (NCI), and Hirshfeld surface analyses.


 Please wait while we load your content...
Please wait while we load your content...