Synthesis and preliminary anti-inflammatory activity exploration of novel derivatives of kirenol†
Abstract
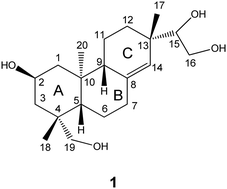
It has been shown in many studies that kirenol is a diterpene with significant biological activity. In this study, thirty-one kirenol derivatives were synthesized. The structures of thirty-one kirenol derivatives were established using IR, 1H NMR, 13C NMR, and LC-MS spectroscopic data. Among them, twenty-nine kirenol derivatives were evaluated for their cytotoxic activities and anti-inflammatory activities. In MTT analysis, all tested compounds showed weak cytotoxicity. Cultured macrophage RAW 264.7 cells were used for the anti-inflammatory experiments. Griess assay was used to evaluate the inhibitory effect of all tested compounds on the overproduction of nitric oxide (NO). The preliminary bioassay test suggested that all of the kirenol derivatives showed strong anti-inflammatory activity. In particular, seven compounds showed better activities than the positive control. We believe that these compounds have greater potential for future druggability and improvement of human health.


 Please wait while we load your content...
Please wait while we load your content...