Enantiomeric separation and molecular docking study of seven imidazole antifungal drugs on a cellulose tris-(3,5-dimethylphenylcarbamate) chiral stationary phase
Abstract
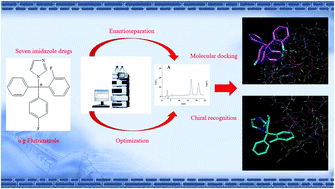
The enantiomeric separation of seven imidazole antifungals was first systematically examined on a derivative polysaccharide chiral stationary phase (CSP), i.e. Chiralcel OD-RH in the reversed phase mode. The influence of the type and content of the organic modifier, the pH of buffer solution, type and concentration of buffer salt, and column temperature on enantiomeric separation were evaluated and optimized. The maximum resolution values for flutrimazole, econazole, miconazole, butoconazole, isoconazole, sulconazole, and climbazole were 2.36, 1.55, 0.89, 1.51, 0.96, 1.13, and 2.30. For a better understanding of the chiral recognition mechanism, detailed computational simulations of Chiralcel OD-RH interaction with seven pairs of enantiomers were carried out using the molecular docking software AutoDock. The various interactions affecting enantiomeric separation were confirmed in detail from the the molecular level. The modeling data were in agreement with the chromatographic results concerning enantioselectivity. It was found that the enantiomeric separation of imidazole analytes on Chiralcel OD-RH was the result of a mixture of hydrogen bonds, π–π, hydrophobic, dipole–dipole, and some special interactions. The docking results showed good agreement with our experimental results.


 Please wait while we load your content...
Please wait while we load your content...