Gas adsorption properties (N2, H2, O2, NO, NO2, CO, CO2, and SO2) on a Sc2CO2 monolayer: a first-principles study
Abstract
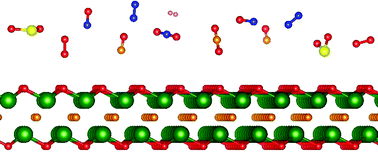
In this work, we study the adsorption properties of N2, H2, O2, NO, NO2, CO, CO2, and SO2 on a Sc2CO2 monolayer based on first-principles calculations from density functional theory. The structural properties of the Sc2CO2 monolayer after adsorption of various gases have been elucidated. The preferred adsorption sites for each gas are determined. It has been established that the CO2, CO, H2 and N2 molecules are physically adsorbed on the Sc2CO2 monolayer, while the NO, SO2, NO2, and O2 molecules are chemically adsorbed on the Sc2CO2 monolayer. The chemical adsorption of O2 molecules, which are a fundamental component of air, significantly changes the electronic properties of the Sc2CO2 monolayer. This fact limits the prospect of application of the Sc2CO2 monolayer in gas sensors or capture of toxic gases such as SO2 and NO.


 Please wait while we load your content...
Please wait while we load your content...