Design, synthesis and biological evaluation of novel pteridinone derivatives as potent dual inhibitors of PLK1 and BRD4
Abstract
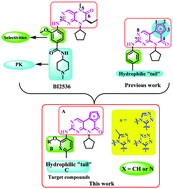
To develop novel simultaneous inhibition of PLK1 and BRD4 bromodomain by a single molecule, three series of novel pteridinone derivatives were designed, synthesized and evaluated for their biological activity. Most compounds exhibited moderate to excellent cytotoxic activity against A549, HCT116, PC-3 and MCF-7 cell lines. The most promising compound III4 showed high antiproliferative effects on four cell lines with IC50 values of 1.27 μM, 1.36 μM, 3.85 μM and 4.06 μM, respectively. The enzymatic assay identified III4 as a potent PLK1 and BRD4 dual inhibitor with % inhibition values of 96.6 and 59.1, respectively. Furthermore, to clarify the anticancer mechanism of the target compounds, explorations in the bioactivity were conducted. The results showed that compound III4 obviously inhibited the proliferation of HCT-116 cell lines, induced a great decrease in the mitochondrial membrane potential leading to apoptosis of cancer cells, suppressed the migration of tumor cells, and arrested the S phase of HCT116 cells.


 Please wait while we load your content...
Please wait while we load your content...