Identification of morpholine based hydroxylamine analogues: selective inhibitors of MARK4/Par-1d causing cancer cell death through apoptosis†
Abstract
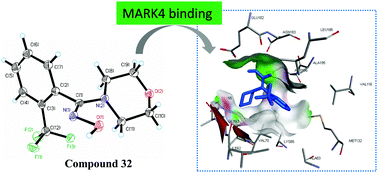
Microtubule affinity-regulating kinase 4 (MARK4) is a serine/threonine kinase involved in the phosphorylation of MAP proteins that regulates microtubule dynamics and abets tumor progression by participating in oncogenic signaling pathways. It is overexpressed in multiple human malignancies and no drug is available for this potential therapeutic target at present. Therefore, using the structure based drug design strategy, a library of hydroxylamine derivatives of morpholine were designed and synthesized as small molecule inhibitors of MARK4. Compound 32 having the CF3 group at the ortho position of the phenyl ring tethered with the >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOH core and the hinge binder morpholine component was found to be a potent and selective inhibitor of MARK4 over thirty other serine-threonine kinases. Study of cell viability and compound induced morphological changes in MCF-7 cancer cells discovered that molecule 32 caused death of cancerous cells through the mechanism of apoptosis. Compound 32 may be transported and delivered to the target site through the blood stream, and has promising antioxidant potential. Such bio-active molecules could serve as optimized lead candidates in drug discovery for cancer treatment through MARK4 inhibition.
NOH core and the hinge binder morpholine component was found to be a potent and selective inhibitor of MARK4 over thirty other serine-threonine kinases. Study of cell viability and compound induced morphological changes in MCF-7 cancer cells discovered that molecule 32 caused death of cancerous cells through the mechanism of apoptosis. Compound 32 may be transported and delivered to the target site through the blood stream, and has promising antioxidant potential. Such bio-active molecules could serve as optimized lead candidates in drug discovery for cancer treatment through MARK4 inhibition.


 Please wait while we load your content...
Please wait while we load your content...