The oxygen reduction reaction of two electron transfer of nitrogen-doped carbon in the electro-Fenton system†
Abstract
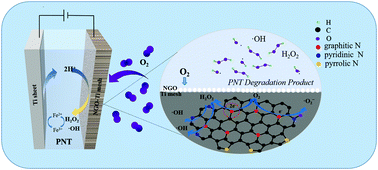
As one of the most important electrochemical advanced catalytic oxidation technologies, the electro-Fenton (EF) method plays an important role in the treatment of refractory organic pollutants in water. The basic reaction principle of the EF reaction is that dissolved oxygen generates hydrogen peroxide (H2O2) through a two electron oxygen reduction reaction (ORR) on the surface of a suitable cathode material. This method is cheap, green and clean. In this study, we synthesized N-doped graphene oxide (NGO), and added carbon black and activated carbon as metal-free electrocatalysts into graphene oxide (GO). The characterization of NGO was carried out by using scanning electron microscopy (SEM), Raman spectroscopy, infrared spectroscopy, X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). The specific surface area and pore size of the samples were measured by the nitrogen adsorption method. The existence of the hydroxyl radical (˙OH) was identified by ESR. It was found that the ratio of GO, carbon black and activated carbon of 4 : 1 : 1 was more favorable for the in situ activation of H2O2 to generate ˙OH. Moreover, we also applied the catalyst to the electro-Fenton (EF) system, and studied its electrocatalytic performance in cyclic voltammetry (CV) and the degradation of pollutants.


 Please wait while we load your content...
Please wait while we load your content...