Synthesis of mono/dinuclear rhenium(i) tricarbonyl substituted with 4-mercaptopyridine related ligands: spectral and theoretical evidence of thiolate/thione interconversion†
Abstract
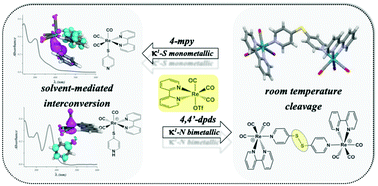
The synthesis, characterization and spectroscopic and theoretical studies of new κ1-S monometallic Re-spy (1)/Re-thiopy (2) and κ1-N-bimetallic Re2-dps (3)/Re2-dpds (4) complexes substituted with 4-mercaptopyridine derivatives are reported. The obtainment of either monosulfide bridge-complex (3) or disulfide bridge-complex (4) depends on the reaction media. The structure of complex 3 was confirmed by monocrystal X-ray diffraction. Additionally it was possible to determine the presence of an intramolecular interconversion between 1 and 2 mediated by solvent molecules through the intermediate species 1-H+ using UV-Vis and NMR spectroscopy. Electronic spectra calculated by TD-DFT feature distinct MLCT transitions for 2 and show that the π-stacking interaction present in 1 is broken in the methanol/water mixture, leading to a structural distribution similar to 2. Cyclic voltammograms of all complexes display the characteristic reduction process for the rhenium center and the bipyridine ligand. Additionally, an irreversible process associated with the bridging ligand for 3 and 4 is observed.


 Please wait while we load your content...
Please wait while we load your content...