Shape-controlled MnO2 as a sulfur host for high performance lithium–sulfur batteries†
Abstract
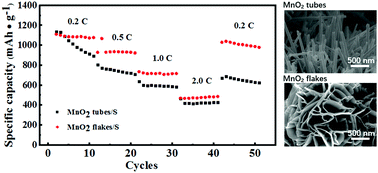
The three-dimensional (3D) porous network structure self-assembled from birnessite-type MnO2 flakes and the urchin-like structure composed of MnO2 nanotubes were fabricated by a convenient one-step hydrothermal method by changing the amount of HCl as the sulfur scaffold for lithium–sulfur batteries. The perfect 3D interconnected nanostructure as a sulfur host displayed excellent electrochemical performance compared to the MnO2 tubes. Benefiting from the well-interconnected 3D spherical architecture, the MnO2/S cathode can reach a high specific capacity of 1582.9 mA h g−1 at 0.1C and a preferable cycling stability with excellent Coulombic efficiency. The porous 3D architecture and the interaction of the Mn–S chemical bond can not only facilitate electron transport, lithium diffusion, and relieve the volume expansion of S but can also effectively restrict the shuttling effect of soluble polysulfides generated during the charge–discharge process.


 Please wait while we load your content...
Please wait while we load your content...