P2S2-Bridged binuclear metal carbonyls from dimerization of coordinated thiophosphoryl groups: a theoretical study†
Abstract
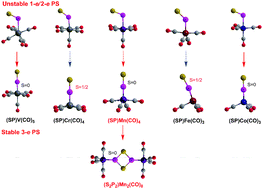
The thiophosphoryl complexes (PS)M(CO)n (M from V to Co) and their corresponding dimers with P2S2 ligands have been studied at the DLPNO-CCSD(T)/cc-pVTZ//M06L/cc-pVTZ level of theory. For the mononuclear complexes containing the highly bent 1-e donor PS group with ∠SPM angles of ∼115°, the (SP)V(CO)6 and (SP)Co(CO)4 complexes are energetically disfavored and (SP)Mn(CO)5 is only slightly favored towards CO loss. The (SP)Cr(CO)5 and (SP)Fe(CO)4 complexes with a bent 2-e donor PS group with ∠SPM angles of ∼133° are both thermodynamically and kinetically disfavored towards dimerization. Similarly all of the (SP)M(CO)n (M from V to Co) complexes with linear 3-e donor PS ligands are energetically disfavored towards dimerization, excepting for Mn(CO)4(PS). The trans-influence of PS follows the sequence 3-e NO ≥ PS (linear) > CS > PS (2-e bent) ≈ CO.


 Please wait while we load your content...
Please wait while we load your content...