Classifying the chemical bonds involving the noble-gas atoms†
Abstract
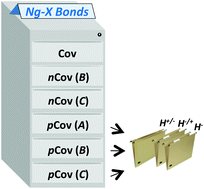
We propose here a general classification of the Ng–X bonds (Ng = noble-gas atom; X = binding partner) that relies on the combined analysis of the local electron energy density H(r), and the reduced density gradient (RDG). Based on the plotted shape of H(r), the Ng–X bonds are first distinguished into types A, B, or C. Based on the topology of H(r), and its integration over the RDG isosurface associated with the bond, any Ng–X bond is then assigned as covalent (Cov), non-covalent (nCov), or partially-covalent (pCov), and any pCov bond is further assayed in terms of the contribution of covalency (H+/−, H−/+, or H−). The method was developed by examining 47 reference species (indicated here as the NgBC47 reference set) of quite diverse bonding character. The obtained classification is, seemingly, robust with respect to the computational level, and of general applicability, as suggested also by test calculations involving some helium, neon, and argon compounds of peculiar bonding character.


 Please wait while we load your content...
Please wait while we load your content...