A copper(i) bromide organic–inorganic zwitterionic coordination compound with a new type of core: structure, luminescence properties, and DFT calculations†
Abstract
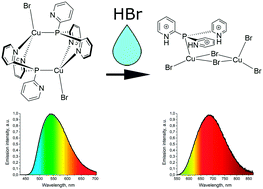
A new organic–inorganic Cu(I) bromide complex based on triple protonated tris(2-pyridyl)phosphine (HPy)3P, [(HPy)3PCu2Br5] (1), has been synthesized by a straightforward reaction in solution or by modification of the [Cu2Br2(Py3P)2] complex using hydrobromic acid. At 77 K, the solid complex 1 exhibits near infra-red photoluminescence with λmax = 680 nm related to 3(M + X)LCT. The emission of 1 rapidly decreases with increasing temperature and is absent at 200 K due to thermal quenching with a small energy barrier ΔE ≈ 590 cm−1. The photophysical results are in good agreement with the TD-DFT calculations showing that in the ground state the cuprophilic interaction between copper ions is absent in spite of the short Cu⋯Cu distance. After annealing of complex 1 at 120 °C, photoluminescence occurred with λmax = 640 nm at 300 K. Complex 1 exhibits a luminescence response in ammonia gas consisting of the appearance of luminescence with λmax = 520 nm followed by the gradual enhancement of the luminescence intensity.


 Please wait while we load your content...
Please wait while we load your content...