A tetrathiafulvalene–l-glutamine conjugated derivative as a supramolecular gelator for embedded C60 and absorbed rhodamine B†
Abstract
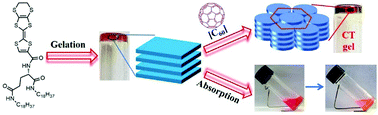
Supramolecular low molecular weight gels made from tetrathiafulvalene derivatives have attracted great interest for various applications. In this work, a novel organic gelator derived from tetrathiafulvalene containing an L-glutamine moiety was designed and synthesized, and the electron-donating property and self-assembly behavior of the gelator were studied. It could form a charge-transfer complex with F4TCNQ in solution. And the gelator showed good gelation properties in alkanes, aromatic solvents and CH2Cl2. The morphologies and structures of the formed gels were characterized by field-emission scanning electron microscopy and X-ray diffraction studies, and the results of FT-IR, 1H NMR and UV-Vis spectroscopy revealed that hydrogen-bonding and π–π interactions played important roles in the gelation process. Furthermore, the gel–sol transition could be regulated by chemical redox, in which the reversibility of the sol–gel transition depended directly on the nature of the gelatinized solvents. Interestingly, the gelator mixed with C60 could form a charge-transfer complex gel in toluene, and the gel–sol transition temperature was observed to increase gradually when adding up to one equivalent of C60 to the gelator. In addition, the gels obtained from toluene and n-hexane showed the ability to absorb the toxic dye rhodamine B from water, and this property suggests the potential utilization for the removal of toxic dye molecules from wastewater.


 Please wait while we load your content...
Please wait while we load your content...