A new electrochemical sensor for the detection of fentanyl lethal drug by a screen-printed carbon electrode modified with the open-ended channels of Zn(ii)-MOF
Abstract
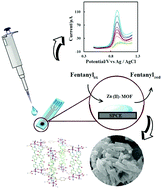
Fentanyl is a potent, effective analgesic and narcotic drug widely used for anesthesia and chronic pain control. In this study, a simple electrochemical method for the detection of fentanyl in aqueous solutions was developed. The modification of a screen-printed carbon electrode (SPCE) was performed by casting a metal–organic framework (MOF) on its surface. The characterization of the zinc-based MOF (Zn(II)-MOF) modifier was investigated via scanning electron microscopy (SEM), thermogravimetric analysis (TGA), Fourier transform infrared (FT-IR) and X-ray diffraction (XRD) techniques. The differential pulse voltammetry (DPV) technique was used for evaluating the fentanyl electrochemical behavior on the electrodes. The optimum experimental conditions were investigated by examining the effects of the scan rate and pH on the cyclic voltammetry (CV) and DPV responses, respectively. The results showed that fentanyl has an irreversible behavior at the potential of 0.9 V and its current increases in the presence of MOF. The application of the presented electrode with the DPV method showed a detection limit of 0.3 μM in the concentration range of 1–100 μM (linear range) for the fentanyl in an aqueous solution. The modified electrode was successfully used to determine the low levels of fentanyl in urine and plasma as the real samples.


 Please wait while we load your content...
Please wait while we load your content...