Exploring functionalized titania for task specific application of efficient separation of trivalent f-block elements†
Abstract
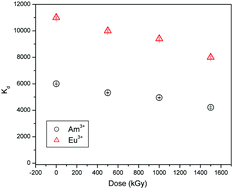
Functionalized titania, obtained by grafting the dipicolinic acid functionality, was explored for task specific application of highly efficient separation of trivalent f-block elements. The sorption mechanism, kinetics and complexation of trivalent f-block elements with the functionalized adsorbent have been investigated in detail. The chemisorption followed the Langmuir isotherm and pseudo 2nd order rate kinetics. The Ti-2p3/2, O-1s and C-1s peaks in the XPS spectra indicate the augmentation of functionalization, while the Eu-4d peak at 136.5 eV confirms the complexation. EXAFS and XANES spectra for Eu L3-edge and Ti K-edge have been explored to characterize the sorbent and its complexation with Eu3+/Am3+. The peak at 1.9 Å was assigned to two oxygen coordination cells at 2.19 Å and 2.39 Å with approximately two and eight oxygen atoms, respectively, whereas the peak at 2.5 Å relates to the coordination peak for Eu:DPA. The sorbent was found to have high radiological stability. DFT calculations optimized the structures of Eu3+/Am3+ in complexation with functionalized titania as M(NO3)3·L species. The Gibbs energy for aqueous phase complexation of Eu3+ and Am3+ was found to be −135.1 and −129.9 kcal mol−1, respectively. The photoluminescence investigation of the Eu–DPA–TiO2–APTES complex revealed the presence of single species without any water molecule in the primary coordination sphere of Eu3+.


 Please wait while we load your content...
Please wait while we load your content...