Experimental and theoretical investigation on MoS2/MXene heterostructure as an efficient electrocatalyst for hydrogen evolution in both acidic and alkaline media†
Abstract
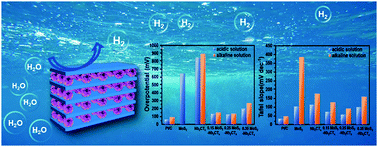
Herein, in order to overcome the poor conductivity and pH limitation of MoS2 in electrocatalytic applications, a MoS2/Nb2CTx heterostructure was synthesized for the first time via an easy hydrothermal method and first-principles investigation was further carried out. The MoS2/Nb2CTx contact shows a metallic property, which guarantees excellent conductivity. Meanwhile, the low p-type Schottky barrier contact on the interface leads to efficient charge transfer from Nb2CTx to MoS2 and further improves its ability to adsorb hydrogen. As a result, the composite showed a low overpotential of 127 mV at 10 mA cm−2, Tafel slope of 56.3 mV dec−1 in acidic solution and an overpotential of 138.8 mV at 10 mA cm−2, and Tafel slope of 93.4 mV dec−1 in alkaline solution. In both solutions, it showed outstanding stability after 3000 cycles. Both experimental and theoretical investigation in our work revealed the potential of this composite for hydrogen evolution reaction in both acidic and alkaline media and provided an effective route to design high-performance MoS2-based catalysts.


 Please wait while we load your content...
Please wait while we load your content...