Vinylimidazole coordination modes to Pt and Au metal centers†
Abstract
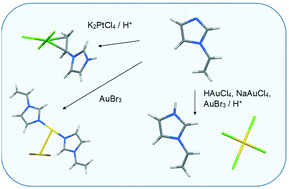
The coordination modes of 1-vinylimidazole to platinum and gold were studied. Complexes [PtCl3(Hvinylimidazole)]·H2O (1), [Au(vinylimidazole)2]+[AuBr2]− (2), [Hvinylimidazole]+[AuCl4]− (3), and [Hvinylimidazole]+[AuBr4]− (4) were prepared and structurally characterized. Compound 1 is the first structurally characterized transition metal complex containing a protonated vinylimidazole, which is coordinated through the vinyl group in the side-on position. In compound 2, the neutral ligands coordinate through the imidazole nitrogens to the reduced gold(I) center and the charge balancing counter anion [Au(I)Br2]− has a short Au–Au contact with the cationic part. In 3 and 4, the acidic reaction conditions lead to the protonation of the imidazole nitrogen and an ion pair with tetrahalogenide gold(III) is obtained. The tendency to the different crystallized products is attributed to the combination of the metal and the halogen properties with the reaction conditions. Computational chemistry was used to explain the preference of the vinyl coordination type, as well as in the interpretation of the spectroscopic details and the nature of the intra- and intermolecular interactions present in the solid state.


 Please wait while we load your content...
Please wait while we load your content...