A gold–carbon dots nanoprobe for dual mode detection of ketamine HCl in soda drinks†
Abstract
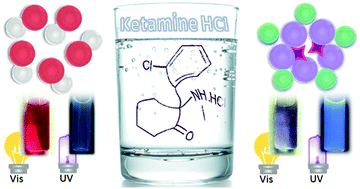
This work describes a dual-mode colorimetric and fluorometric nanoprobe for determination of ketamine hydrochloride (KET) in soda beverages. KET is a widely known club drug that is spiked in beverages to get rape victims vulnerable. The proposed nanoprobe utilizes gold nanoparticles (AuNPs) and fluorescent carbon dots (CDs). AuNPs act as a color probe and quencher of CDs fluorescence. KET destabilizes the citrate-capped AuNPs leading to a color change and subsequent disruption of the inner filter quenching mechanism in this acceptor/donor pair. Nanoparticles are characterized by transmission electron microscopy as well as molecular absorption and emission spectroscopy. The dual-detection mode has been optimized for the concentration of nanoparticles, reaction time and medium pH. The fluorescence recovery efficiency of CDs and the absorbance ratio of AuNPs bands are used as analytical signals for dual detection of KET. A linear response in the range of 5 × 10−5–6.5 × 10−4 mol L−1 is achieved for the fluorescent sensor, with a detection limit of 2.32 × 10−5 mol L−1. The respective values for the color sensor are 1 × 10−4–9 × 10−4 and 2.70 × 10−5 mol L−1. The selectivity of this dual-mode nanoprobe has been investigated by application to different spiked soda drinks. The sensor exhibits good selectivity towards Sprite® and Coca-Cola® in the average oral KET dose; however, the orange coloration in Fanta® seems to have high absorbance over the AuNPs characteristic band that hinders the detection of KET at the average concentration level.


 Please wait while we load your content...
Please wait while we load your content...