Reduction chemistry of hexagonal boron nitride sheets and graphene: a comparative study on the effect of alkali atom doping on their chemical reactivity
Abstract
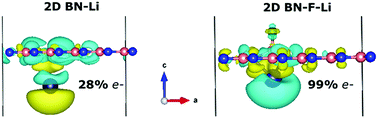
By means of first-principles calculations, we investigate the reduction chemistry of monolayer boron nitride (2D BN) and graphene. The adsorption of alkali atoms dramatically increases the reactivity of 2D BN, Li being the most powerful agent, closely followed by K and Na. Interestingly, the reactivity enhancement in 2D BN is 3.6 times larger in comparison with graphene. On average, Li adsorption enhances the adsorption energies by 66.0 and 18.2 kcal mol−1 for 2D BN and graphene, respectively. The higher increment in adsorption energies observed for 2D BN can be attributed to the significant difference in alkali charge donation to pristine and functionalized 2D BN. In contrast, for graphene, this difference is much smaller. The increase of reactivity strongly depends on the functional group added as well as on the alkali used. The reduction chemistry facilitates the addition of functional groups to 2D BN which cannot be added without the presence of alkalis. We conclude that reduced 2D BN is more reactive than reduced graphene when alkalis act as reducing agents. We expect that this work will motivate new investigations to attain the covalent functionalization of 2D BN.


 Please wait while we load your content...
Please wait while we load your content...