Multicomponent synthesis of diphenyl-1,3-thiazole-barbituric acid hybrids and their fluorescence property studies†
Abstract
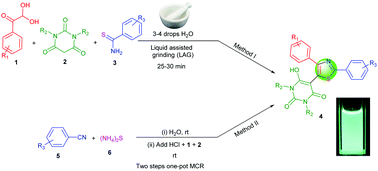
A series of novel diphenyl-1,3-thiazole linked barbituric acid hybrids (4) were prepared by two catalyst-free methods from readily available starting materials. The reaction of arylglyoxal, barbituric acid and aryl thioamides in the presence of 3–4 drops of water and liquid assisted grinding (LAG) provides the corresponding trisubstituted thiazoles tethered with a barbituric acid moiety within 30 minutes. Alternatively, a sequential two-step one-pot process involving aryl nitriles, ammonium sulphide, arylglyoxal and barbituric acid in water medium was developed. In this second method, in situ thioamides were prepared at room temperature from the reaction of alkyl/aryl nitriles and ammonium sulphide in aqueous medium. Arylglyoxal and barbituric acid were added to the in situ thioamides after neutralizing the reaction medium to provide trisubstituted thiazoles linked with barbituric acid derivatives. Some of our synthesized molecules showed fluorescent properties with very good quantum yields in DMSO medium. We also observed that fluorescent quantum yields of these thiazole derivatives depend on the type of electron donating/withdrawing character of R1 and R3. R2 has a very small effect on tuning the fluorescent properties. The salient features of this work are catalyst-free reactions, wide substrate scope, green reaction conditions (liquid assisted grinding and room temperature reactions in water medium) as well as the presence of more than one pharmaceutically important heterocyclic moiety with fluorescent properties.


 Please wait while we load your content...
Please wait while we load your content...