Synthesis of chiral iron-based ionic liquids: modelling stable hybrid materials†
Abstract
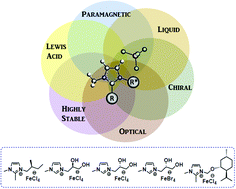
Five chiral iron-containing ionic liquids were synthetized from several chiral ionic liquids (CILs) based on different imidazolium cations. These novel compounds were prepared through an easy synthetic method involving mixing 1 equivalent of iron salt FeX3 (X = Cl or Br) and 1 equivalent of chiral ionic liquid. The so-obtained chiral iron-based ionic liquids contain easily tunable imidazolium cations, which allow the preparation of a wide range of compounds within different functional groups and chiral moieties. These chiral iron-based ionic liquids have been fully characterized by a wide variety of techniques, including polarimetry, inductively coupled plasma optical emission spectrometry, elemental analysis, thermogravimetric analysis, differential scanning calorimetry, magnetic susceptibility measurements, and UV-vis, attenuated total reflection Fourier-transform infrared and Raman spectroscopies. Such experiments confirmed the structure of these new materials, as well as their appealing properties. The combination of a chiral moiety with a haloferrate anion within the IL structure allows the formation of very stable and multifunctional compounds with promising chiral, magnetic, optical and acidic properties. The resulting chiral iron-based ILs exhibit great potential for use in a range of applications such as enantioselective catalysis or chiral recognition, to name a few.


 Please wait while we load your content...
Please wait while we load your content...