A facile solvothermal synthesis of Pt1.2Co/C bimetallic nanocrystals as efficient electrocatalysts for methanol oxidation and hydrogen evolution reaction†
Abstract
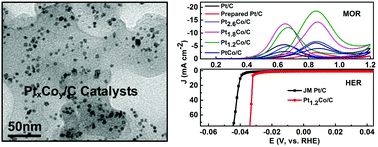
Alloying Pt with less expensive 3d-transition metals is effective for increasing the electrocatalytic activity and CO tolerance while decreasing Pt consumption. Herein, well-dispersed and highly alloyed Pt–Co bimetallic nanoparticles supported on commercial carbon black have been synthesized by means of a facile one-pot solvothermal approach without using any bulky capping agents. The best performing Pt1.2Co/C, with an average particle size of 4.7 ± 0.8 nm, has a large electrochemically active surface area of 64.30 m2 g−1, which is comparable to that of 66.73 m2 g−1 of commercial Pt/C and larger than that of other synthesized catalysts. The as-synthesized Pt1.2Co/C shows about 3 times enhanced mass activity and specific activity for methanol oxidation reaction compared to commercial Pt/C (20 wt%). Meanwhile, the electrocatalytic performance of Pt1.2Co/C for hydrogen evolution reaction (HER) in acid media is also superior to commercial Pt/C and the overpotential is only 33.8 mV when the current density reaches 70 mA cm−2. This work demonstrates that the developed strategy provides a facile platform for the synthesis of high-performance, low-cost and robust catalysts in practical catalysis, and energy storage and conversion.


 Please wait while we load your content...
Please wait while we load your content...