Effect of surface acidity on the catalytic activity and deactivation of supported sulfonic acids during dehydration of methanol to DME
Abstract
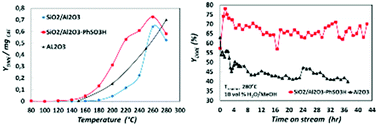
The influence of a catalyst's surface acidity on the catalytic activity and deactivation in the dehydration of methanol to DME was investigated. Different materials including propylsulfonic acid functionalized silica with different Brønsted acidities, silica–alumina, and propyl and phenylsulfonic acid functionalized silica–alumina catalysts were prepared. All the samples were characterized by XRD, TGA, XPS, N2-sorption, ICP-OES and SEM analysis. It was found that the Brønsted and Lewis acidity of the SiO2/Al2O3-PhSO3H catalyst played a critical role in the performance of methanol to DME transformation. The grafting of sulfonic acid groups on silica–alumina enhanced the surface Brønsted acidity and also the reaction activity and selectivity for the dehydration of methanol to DME. In addition, the phenylsulfonic acid functionalized silica–alumina catalyst exhibited the highest activity and stability for the dehydration reaction at relatively low temperatures at which the γ-Al2O3 catalyst, commercial reference, displayed low dehydration activity. The effect of water was also investigated because in the indirect process to produce DME using acidic γ-alumina, it had the most important effect on catalyst deactivation. As a result, water had a positive effect on methanol dehydration over the SiO2/Al2O3-PhSO3H catalyst in contrast to γ-Al2O3 which was rapidly deactivated. Thus, Brønsted and Lewis acid sites with suitable strength may be responsible for the effective conversion of methanol to DME with high stability and selectivity.


 Please wait while we load your content...
Please wait while we load your content...