Metal-free annulative hydrosulfonation of propiolate esters: synthesis of 4-sulfonates of coumarins and butenolides†
Abstract
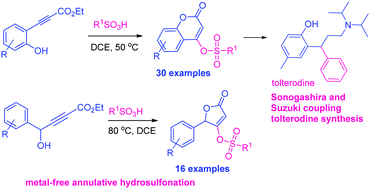
An efficient metal-free and cost-effective method for the synthesis of coumarin and butenolide 4-sulfonates (46 examples) has been developed. The reaction involves addition of sulfonic acids to ethyl propiolates followed by lactonization, resulting in direct formation of coumarin and butenolide 4-sulfonates. This methodology has been elaborated to Sonogashira and Suzuki coupling including the synthesis of rac-tolterodine.


 Please wait while we load your content...
Please wait while we load your content...