The anionic recognition mechanism based on polyol and boronic acid receptors†
Abstract
Anion selective recognition plays pivotal roles in the environmental field. The polyol and boronic acid receptor classes are investigated here to recognize the chloride (Cl−); fluoride (F−); dihydrogen phosphate (H2PO4−); acetate (AcO−); bromide (Br−); and hydrogen sulfate (HSO4−) anions. Energy Decomposition Analysis (EDA) shows that the anion–receptor interactions are predominantly electrostatic. Interestingly, the increase of the number of –CF3 groups or of the carbonic chain in the polyol structure favors Cl− recognition. The polyol–Cl− interactions are more attractive than the boronic acid–Cl− bond. Boronic acid recognizes F− and H2PO4− with the most attractive energies. The Quantum Theory of Atoms in Molecules (QTAIM) method elucidated that the recognition of AcO−, Cl−, Br−, and HSO4− by the receptors is maintained through hydrogen bonds, while the recognition of F− and H2PO4− by boronic acid involves B⋯F− and B⋯O−–P bonds, respectively, showing a larger covalent character compared to other boronic acid–anion bonds, but with a primarily electrostatic nature.
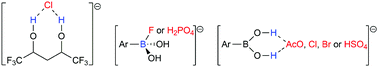


 Please wait while we load your content...
Please wait while we load your content...