Chromogenic agents built around a multifunctional double-triazine framework for enzymatically triggered cross-linking under physiological conditions†
Abstract
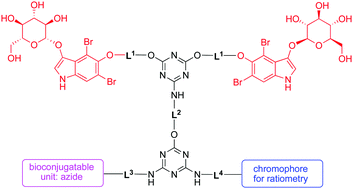
An “A2BC”-type bioconjugatable compound (1) that enables enzymatically triggered cross-linking chemistry has been designed and synthesized. The compound contains two indoxyl-glucoside units (A units), which upon action of a glucosidase undergo oxidative dimerization to form a water-insoluble indigoid dye. The other two moieties are azide (B, for click chemistry) and (C) a triazine-chloride or a coumarin dye; the former enables substitution with a nucleophile while the latter provides for ratiometric sensing of the extent of indigoid dye formation. A 7-component building block synthesis relies on successive substitution of 2 cyanuric chloride (2,4,6-trichloro-1,3,5-triazine) molecules with 5 other constituents (2A, B, C, and a piperazine–sulfobetaine moiety); the latter bear oligoethylene glycol (i.e., PEG) or aminoalkyl tethers. The substitution reactions of cyanuric chloride proceed almost entirely at room temperature with control achieved by the order of nucleophile reactivity (aliphatic amine > phenol ≫ aliphatic alcohol) and the nature of a chosen base. Treatment of 1 with glucosidase under physiological conditions in aqueous solution gave an indigoid solution and a precipitate (∼1 : 9 ratio) that were characterized by absorption spectrometry (including multicomponent analysis), dynamic light-scattering spectroscopy, and optical microscopy. Ratiometric absorption of the integral coumarin dye with the indigoid scaffold showed a low yield (3%) of oligomerization, which suggests future molecular designs might employ longer linkers and/or substituents with greater water solubility.


 Please wait while we load your content...
Please wait while we load your content...