Catalytic activity of Mg–Al hydrotalcites and derived mixed oxides for imination reactions via an oxidative-dehydrogenation mechanism†
Abstract
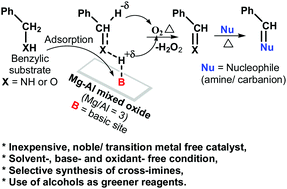
The catalytic activities of Mg–Al hydrotalcites and derived Mg–Al mixed oxides were studied for the imination of benzylic substrates (benzyl amine and benzyl alcohol), with aniline as a model reaction, to establish a green and cost-effective catalytic (precious metal free) process for imination and tandem reactions utilizing alcohols as reagents. The Mg–Al mixed oxide (Mg/Al ratio = 3.0) was found to be an efficient catalyst for imination reactions to synthesize cross-imines with high selectivity as well as for tandem reactions of alcohols. Basic sites of catalysts adsorb benzylic substrates through the hydrogen of their functional groups (–NH2/–OH) and activate (weaken) benzylic C–H bonds for hydride elimination. Thus, basic sites catalyze the reaction by activating benzylic substrates for oxidative-dehydrogenation (i.e., deprotonation followed by hydride elimination with the help of oxygen) to produce reactive imine/carbonyl intermediates, which undergo subsequent nucleophilic reactions with amine.


 Please wait while we load your content...
Please wait while we load your content...