Homoleptic and heteroleptic Zn(ii) selone catalysts for thioetherification of aryl halides without scrubbing oxygen†
Abstract
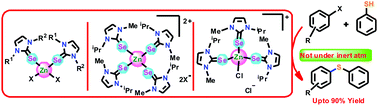
Five new mononuclear tetra-coordinated zinc(II) selones, [Zn(L1)2Cl2] (1), [Zn(L1)2Br2] (2), [{Zn(L2)4}{BF4}2] (3), [{Zn(L2)4}{ClO4}2] (4), and [Zn(L2)2Br2] (5), have been isolated from a one-pot reaction between the corresponding zinc(II) salt and selone ligand, 1-methyl 3-naphthylmethylimidazoline-2-selone (L1) or 1-isopropyl 3-methylimidazoline-2-selone (L2). All these complexes were characterized by CHN analysis, FT-IR, NMR studies, and single-crystal X-ray crystallography techniques. The Zn(II) center in 1–5 exhibits a distorted tetrahedral geometry. Besides, 1–5 were employed as catalysts in the thioetherification of aryl halides. The first zinc(II) catalyst-mediated thioetherification of aryl halides without scrubbing oxygen was demonstrated. Catalysts 1–5 are highly active towards the cross-coupling reaction between aryl halides and thiophenols. The catalytic ability of 1–5 was explored in THF, toluene, and CH3CN solvents with different bases such as K2CO3, Cs2CO3, and NaOtBu. Interestingly, the zinc(II) center attached to two selone ligands is much more catalytically active than that attached to four selone ligands.


 Please wait while we load your content...
Please wait while we load your content...