High protonic conduction in two metal–organic frameworks containing high-density carboxylic groups†
Abstract
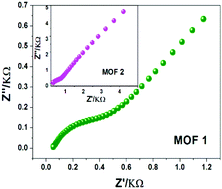
The synthesis of crystalline solid MOFs showing high proton conductivity still faces great challenges. The approach of delicately designing organic functional ligands will be very conducive to the realization of this goal. Herein, by adopting a designed ligand, namely, (p-N-imidazol-1-yl)-phenyl-1H-imidazole-4,5-dicarboxylic acid (p-IPhH3IDC) containing two imidazole and two carboxylate units, two hydrolytically stable 2D MOFs, namely, [Zn(p-IPhHIDC)]n (1) and [Co(p-IPhHIDC)]n (2) with high-density uncoordinated –COOH groups were successfully prepared. The water-assisted proton conducting characteristics of the two MOFs were explored. Expectedly, MOFs 1 and 2 exhibit super-high protonic conductivities of up to 1.9 × 10−3 and 1.07 × 10−3 S cm−1, respectively, at 100 °C and 98% RH, which are higher than those of previously reported MOFs having high proton conductivities. Moreover, their proton-conducting performances were maintained for at least 8 days. We further discussed the conducting mechanism from crystal structural analyses, N2 and H2O vapor adsorption/desorption results and the calculated Ea values and pointed out that the uncoordinated –COOH groups in the frameworks were responsible for the high proton conductivity. Our study provides a useful strategy for constructing MOFs with high proton conductivity by assembling multiple functional units into an organic ligand.


 Please wait while we load your content...
Please wait while we load your content...