Simultaneous detection of paracetamol and 4-aminophenol at nanomolar levels using biocompatible cysteine-substituted phthalocyanine†
Abstract
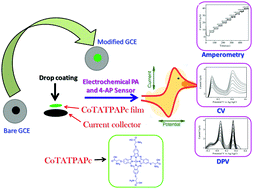
Extension of the conjugation and biocompatibility of the phthalocyanine molecule is expected to improve its stability and interaction with bio-molecules without any fouling. Hence, a novel thio-bridged cysteine ligand and its respective cobalt phthalocyanine complex (CoTATPAPc) have been synthesized. These molecules were characterized with TGA and FT-IR, UV-Vis, mass and NMR spectroscopy techniques. The spectral data reveal the successful formation of the desired compounds with high purity. The CoTATPAPc molecule was uniformly coated on the electrode, and the designed GCE/CoTATPAPc displayed remarkable electrocatalytic efficiency for the simultaneous sensing of paracetamol (PA) and 4-aminophenol (4-AP). The amperometric sensor successfully exhibited a linear response in the 20 to 360 nM concentration range, with LOD values of 4.2 and 4.1 nM for 4-AP and PA, respectively. The DPV technique displayed LODs of 6.1 and 6.3 nM with sensitivity values of 0.2211 and 0.0197 μA nM−1 cm−2 for 4-AP and PA, respectively. The sensitivity value of GCE/CoTATPAPc was extremely high, indicating the superior and rapid response of the designed sensor. This sensor showed great reproducibility, repeatability, high selectivity and stability for detecting PA as well as 4-AP even when interfering compounds were present in high concentrations. The developed sensor was successfully evaluated for the testing of commercial samples.


 Please wait while we load your content...
Please wait while we load your content...