A biscoumarin scaffold as an efficient anti-Zika virus lead with NS3-helicase inhibitory potential: in vitro and in silico investigations†
Abstract
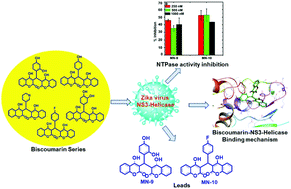
As a way to investigate new antiviral leads against Zika virus (ZIKV), we have targeted the NS3 helicase (NS3H) protein with biscoumarin derivatives. The NS3H protein from ZIKV is important, as it plays a significant role in the viral genome replication process. We have assessed the NTPase modulatory effects of biscoumarin derivatives against the NTPase activity of NS3H through in vitro enzymatic studies. Subsequently, to explore the mechanism, detailed computational studies were conducted. The NS3H protein has two binding cavities: one is the NTPase binding cavity where ATP binds, and the other is an RNA binding cavity for the binding of RNA molecules. Biscoumarin derivatives were found to be efficient in binding to both cavities, i.e., the NTPase and RNA binding cavities of the NS3H protein. A biscoumarin derivative with the best binding affinity for the NTPase binding cavity and the lowest binding affinity for the RNA binding groove revealed the best in vitro NTPase inhibitory activity. Also, the biscoumarin derivative with the lowest binding affinity for the NTPase binding cavity and higher binding affinity for the RNA binding cavity revealed the worst NTPase inhibitory activity under the same in vitro assay conditions. Here, we concluded that biscoumarin derivatives are modulators of the NS3H protein and they can be considered as promising anti-viral lead molecules. The structural activity relationships (SARs) of the biscoumarin derivatives in relation to their NTPase inhibitory activities were established, and we reported two derivatives, namely MN-9 and MN-10, as potential NS3H–NTPase inhibitor molecules.


 Please wait while we load your content...
Please wait while we load your content...