End-group functionalization of a conjugated azomethine with ureas for property tailoring†
Abstract
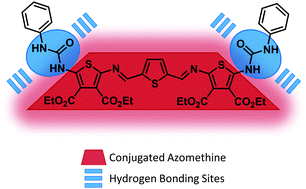
A conjugated azomethine consisting of three thiophenes, two azomethine bonds, and end-terminated with phenyl-ureas was prepared by a convergent approach. Single crystal X-ray crystallography confirmed intermolecular hydrogen bonding with one of the terminal ureas to form a supramolecular dimer. Hydrogen bonding was also confirmed by FT-IR, which was complemented by theoretical calculations. Three colored states were observed, one each for the electrochemically oxidized and reduced states in addition to the neutral state. The urea substituted termini also contributed to a cathodic behavior, leading to an ambipolar character of the conjugated azomethine. The fluorescence of the red colored conjugated azomethine was enhanced by activating hydrogen bonding involving the ureas, either with protic solvents or by self-association in a thin film. The fluorescence could also be enhanced in frozen matrices of 2-methyl-THF and a mixture of ethanol/methanol.


 Please wait while we load your content...
Please wait while we load your content...