Synthesis and biological activity of imidazole based 1,4-naphthoquinones†
Abstract
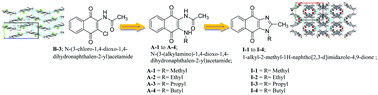
Design and development of drugs in multi-drug resistant (MDR) infections have been of growing interest. We report the syntheses, and antibacterial and antifungal activities of imidazole-based 1,4-naphthoquinones (I-1 to I-4; 1-alkyl-2-methyl-1H-naphtho[2,3-d]imidazole-4,9-dione (alkyl = methyl to butyl)) and their precursors (B-3; N-(3-chloro-1,-dioxo-1,4-dihydronaphthalen-2-yl)acetamide) and A-1 to A-4; N-(3-(alkylamino)-1,4-dioxo-1,4-dihydronaphthalen-2-yl)acetamide (alkyl = methyl to butyl). Crystal structures of B-3, A-1 to A-3 and I-2 to I-4 were obtained through single crystal X-ray diffraction experiments. Electronic structure and charge distribution have further been characterized with the use of Density Functional Theory. Seven of these derivatives display a broad spectrum of antibacterial activity against few selected bacterial strains (Gram-positive and Gram-negative). As demonstrated MIC values with B-2 and B-3 against bacterial isolates were 8–64 μg ml−1 and those against pathogenic yeast, C. albicans, were observed in the range of 128–256 μg ml−1. MIC data of these derivatives suggest them to be promising against pathogens.


 Please wait while we load your content...
Please wait while we load your content...