Prediction of two-dimensional CP3 as a promising electrode material with a record-high capacity for Na ions†
Abstract
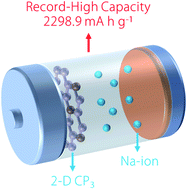
Borophene with a maximum Li/Na capacity of 1984 mA h g−1 (nanoscale 2016, 8, 15 340–15 347) has shown the highest capacity among two-dimensional (2-D) anode materials identified so far. Herein, we report the record break for Na-ion using a newly proposed 2-D material, namely, CP3. We fully investigated Li- and Na-ion adsorption and diffusion processes on a CP3 monolayer. We found that the material can enable stable Li/Na adsorption considering charge accumulation on CP3 surfaces. The ion diffusion barriers for Li and Na were identified to be 98 meV and 356 meV, respectively. These values were comparable or smaller than those of the typical high-capacity electrode materials such as borophene. Most remarkably, the maximum Na capacity in CP3 monolayer can reach up to 2298.9 mA h g−1, which breaks the value recorded using borophene (1984 mA h g−1). Our work highly promises that the 2-D CP3 material could serve as an outstanding electrode material for Na-ion batteries with an extremely high storage capacity.


 Please wait while we load your content...
Please wait while we load your content...