Selenium supplementation protects against oxidative stress-induced cardiomyocyte cell cycle arrest through activation of PI3K/AKT†
Abstract
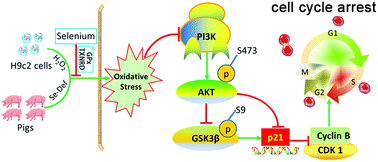
Oxidative stress significantly contributes to heart disease, and thus might be a promising target for ameliorating heart failure. Mounting evidence suggests that selenium has chemotherapeutic potential for treating heart disease due to its regulation of selenoproteins, which play antioxidant regulatory roles. Oxidative stress-induced cardiomyocyte cell cycle arrest contributes to the loss of cardiomyocytes during heart failure. The protective effects and mechanism of selenium against oxidative stress-induced cell cycle arrest in cardiomyocytes warrant further study. H9c2 rat cardiomyoblast cells were treated with hydrogen peroxide in the presence or absence of selenium supplementation. Na2SeO3 pretreatment alleviated H2O2-induced oxidative stress, increased thioredoxin reductase (TXNRD) activity and glutathione peroxidase (GPx) activity and counteracted the H2O2-induced cell cycle arrest at the S phase. These effects were accompanied by attenuation of the H2O2-induced strengthening of the G2/M-phase inhibitory system, including increased mRNA and protein levels of cyclin-dependent kinase 1 (CDK1) and decreased p21 mRNA levels. Notably, Na2SeO3 pretreatment activated the PI3K/AKT signaling pathway, and inhibition of PI3K counteracted the protective effects of selenium on H2O2-induced cell cycle arrest. We corroborated our findings in vivo by inducing oxidative stress in pig heart by feeding a selenium deficient diet, which decreased the TXNRD activity, inactivated PI3K/AKT signaling and strengthened the G2/M-phase inhibitory system. We concluded that the cardioprotective effects of selenium supplementation against oxidative stress-induced cell cycle arrest in cardiomyocytes might be mediated by the selenoprotein-associated (GPx and TXNRD) antioxidant capacity, thereby activating redox status-associated PI3K/AKT pathways, which promote cell cycle progression by targeting the G2/M phase inhibitory system. This study provides new insight into the underlying mechanisms of cardioprotection effects of selenium at the cellular level.


 Please wait while we load your content...
Please wait while we load your content...