Quantitative capillary zone electrophoresis-mass spectrometry reveals the N-glycome developmental plan during vertebrate embryogenesis†
Abstract
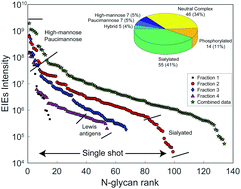
Glycans are known to be involved in many biological processes, while little is known about the expression of N-glycans during vertebrate development. We now report the first quantitative studies of both the expression of N-linked glycans at six early development stages and the expression of N-glycosylated peptides at two early development stages in Xenopus laevis, the African clawed frog. N-Glycans were labeled with isobaric tandem mass tags, pooled, separated by capillary electrophoresis, and characterized using tandem mass spectrometry. We quantified 110 N-glycan compositions that spanned four orders of magnitude in abundance. Capillary electrophoresis was particularly useful in identifying charged glycans; over 40% of the observed glycan compositions were sialylated. The glycan expression was relatively constant until the gastrula–neurula transition (developmental stage 13), followed by massive reprogramming. An increase in oligomannosidic and a decrease in the paucimannosidic and phosphorylated oligomannosidic glycans were observed at the late tailbud stage (developmental stage 41). Two notable and opposing regulation events were detected for sialylated glycans. LacdiNAc and Lewis antigen features distinguished down-regulated sialylation from up-regulated species. The level of Lewis antigen decreased at later stages, which was validated by Aleuria aurantia lectin (AAL) and Ulex europaeus lectin (UEA-I) blots. We also used HPLC coupled with tandem mass spectrometry to identify 611 N-glycosylation sites on 350 N-glycoproteins at the early stage developmental stage 1 (fertilized egg), and 1682 N-glycosylation sites on 1023 N-glycoproteins at stage 41 (late tailbud stage). Over two thirds of the N-glycoproteins identified in the late tailbud stage are associated with neuron projection morphogenesis, suggesting a vital role of the N-glycome in neuronal development.
- This article is part of the themed collection: Glycomics & Glycoproteomics: From Analytics to Function


 Please wait while we load your content...
Please wait while we load your content...