Recent progress toward the asymmetric synthesis of carbon-substituted piperazine pharmacophores and oxidative related heterocycles
Abstract
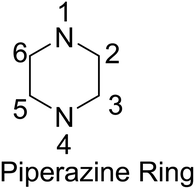
The important requirement for approval of a new drug, in case it happens to be chiral, is that both enantiomers of the drug should be studied in detail, which has led synthetic organic and medicinal chemists to focus their attention on the development of new methods for asymmetric synthesis especially of relevant saturated N-heterocycles. On the other hand, the piperazine ring, besides defining a major class of saturated N-heterocycles, has been classified as a privileged structure in medicinal chemistry, since it is more than frequently found in biologically active compounds including several marketed blockbuster drugs such as Glivec (imatinib) and Viagra (sildenafil). Indeed, 13 of the 200 best-selling small molecule drugs in 2012 contained a piperazine ring. Nevertheless, analysis of the piperazine substitution pattern reveals a lack of structural diversity, with almost every single drug in this category (83%) containing a substituent at both the N1- and N4-positions compared to a few drugs having a substituent at any other position (C2, C3, C5, and C6). Significant chemical space that is closely related to that known to be biologically relevant, therefore, remains unexplored. In order to explore this chemical space, efficient and asymmetric syntheses of carbon-substituted piperazines and related heterocycles must be designed and developed. Initial, recent efforts toward the implementation of this particular target are in fact the subject of this review.


 Please wait while we load your content...
Please wait while we load your content...