Synthesis and in vivo evaluation of a radiofluorinated ketone body derivative†
Abstract
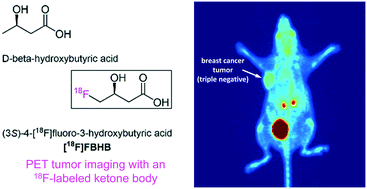
The ketone bodies D-beta-hydroxybutyric acid and acetoacetic acid represent the principal oxidative energy sources of most tissues when dietary glucose is scarce. An 18F-labeled ketone body could be a useful tool for studying ketone body metabolism using positron emission tomography (PET). Here, we report the first radiofluorinated ketone body derivative (3S)-4-[18F]fluoro-3-hydroxybutyric acid ([18F]FBHB) as well as its enantiomer and L-beta-hydroxybutyric acid derivative, (3R)-4-[18F]fluoro-3-hydroxybutyric acid ((R)-[18F]F3HB). PET imaging in mice showed biodistribution profiles of the radiotracers that were consistent with the biodistribution of the respective endogenous compounds. Moreover, both enantiomers visualized breast cancer xenografts in vivo. Fasting over 24 h showed significantly enhanced brain and heart uptake of [18F]FBHB and tumor uptake of (R)-[18F]F3HB. Disorders exhibiting altered energy substrate utilization, such as Alzheimer's disease, epilepsy, diabetes, and cancer may be of interest for PET imaging studies using [18F]FBHB.


 Please wait while we load your content...
Please wait while we load your content...