Conversion of amino acids to aryl/heteroaryl ethanol metabolites using human CYP2D6-expressing live baker's yeast†
Abstract
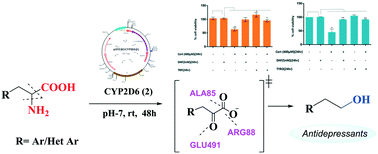
Different natural aromatic/heterocyclic L-amino acids were biotransformed into aryl/heteroaryl ethanol metabolites via oxidative deamination, decarboxylation and reduction cascades using live baker's yeast cells producing intracellular human CYP2D6 enzyme. Among the three yeast strains expressing 3 different CYP2D6 variants, CYP2D6(2) (i.e. CYP2D6 wild-type) provided the best result under neutral pH conditions at RT. We have successfully converted six natural amino acids into their corresponding alcohols, having one carbon atom less, with moderate yields. Some of the downstream products like tryptophol and tyrosol induced the pTrKB (Tropomyosin receptor kinase B) activation pattern similar to that of BDNF (brain-derived neurotrophic factor), thereby depicting potential antidepressant activity. Control experiments and molecular modelling studies revealed that this tandem bio-transformation probably happens via a pyruvate intermediate. This study establishes that CYP2D6-expressing live yeast cells can be a powerful tool for the enzymatic C–N, C–C bond cleavage of amino-acids.


 Please wait while we load your content...
Please wait while we load your content...