Antiproliferative activities of tricyclic amides derived from β-caryophyllene via the Ritter reaction against MDA-MB-231 breast cancer cells†
Abstract
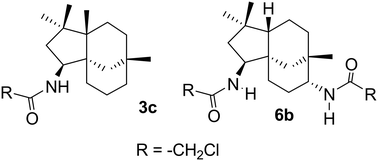
A library of novel tricyclic amides has been synthesised via the Ritter reaction from β-caryophyllene 1 and its monoepoxy derivative 4. The compounds were assessed for antiproliferative activities against the aggressive MDA-MB-231 breast cancer cell line. Of the synthesised compounds, eight were active. 3c and 6b were the most potent and inhibited proliferation with IC50 of 9.7 and 8.2 μM, respectively. Mechanistic studies revealed differences in their antiproliferative actions. 6b inhibited cell cycle progression and induced predominantly apoptotic cell death. In contrast, 3c did not affect cell cycle kinetics and favoured necrotic over apoptotic pathways. Screening against mammalian cells (VERO cells) indicates that 3c and 6b were more active towards MDA-MB-231 cells than noncancerous cells. Facile synthesis and biological results suggest that these caryophyllene derived amides are viable lead compounds for further development.


 Please wait while we load your content...
Please wait while we load your content...