Cyclic siloxanes conjugated with fluorescent aromatic compounds as fluoride sensors†
Abstract
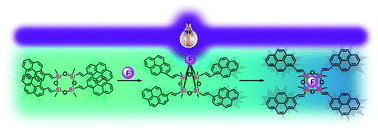
Cyclic siloxanes conjugated with anthracene (D4An) and pyrene (D4Py) were prepared via Heck coupling reactions of 2,4,6,8-tetramethyl-2,4,6,8-tetravinylcyclotetrasiloxane with 9-bromoanthracene and 1-bromopyrene, respectively. These materials exhibited solvent-dependent fluorescence with the largest Stokes shift of up to ∼146 nm, confirming the presence of intramolecular exciplex formation among the fluorophores within a cyclic siloxane. After reacting with fluoride ions, blue-shifted emissions of D4An and D4Py in organic solvents were observed; it suggests an increase in monomer emission as a result of exciplex preclusion via Si–F bond formation. Moreover, the colorless solutions of D4An and D4Py in THF turned pink and orange, respectively, in the presence of excessive F−, which also suggested the formation of charge-transfer complexes. The interactions between cyclic siloxanes and fluoride ions were also studied via FTIR, ESI-MS and NMR experiments. Computational modeling studies revealed that fluoride played an important role in distorting the geometry of cyclic siloxanes by interrupting excimers of fluorescent aromatic rings in the side-chain.
- This article is part of the themed collection: Fluorescent and Luminescent Materials


 Please wait while we load your content...
Please wait while we load your content...