A 96-well format microvascularized human lung-on-a-chip platform for microphysiological modeling of fibrotic diseases†
Abstract
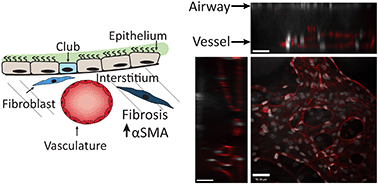
Development of organoids and microfluidic on-chip models has enabled studies of organ-level disease pathophysiologies in vitro. However, current lung-on-a-chip platforms are primarily monolayer epithelial–endothelial co-cultures, separated by a thin membrane, lacking microvasculature-networks or interstitial-fibroblasts. Here we report the design, microfabrication, and characterization of a unique microphysiological on-chip device that recapitulates the human lung interstitium–airway interface through a 3D vascular network, and normal or diseased fibroblasts encapsulated within a fibrin-collagen hydrogel underneath an airlifted airway epithelium. By incorporating fibroblasts from donors with idiopathic pulmonary fibrosis (IPF), or healthy-donor fibroblasts treated with TGF-β1, we successfully created a fibrotic, alpha smooth muscle actin (αSMA)-positive disease phenotype which led to fibrosis-like transformation in club cells and ciliated cells in the airway. Using this device platform, we further modeled the cystic fibrosis (CF) epithelium and recruitment of neutrophils to the vascular networks. Our results suggest that this microphysiological model of the human lung could enable more pathophysiologically relevant studies of complex pulmonary diseases.


 Please wait while we load your content...
Please wait while we load your content...