A novel microfluidic device integrating focus-separation speed reduction design and trap arrays for high-throughput capture of circulating tumor cells†
Abstract
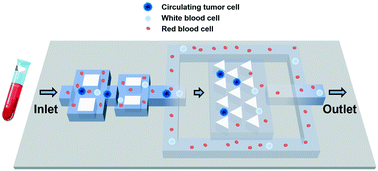
Isolation and analysis of circulating tumor cells (CTCs) from peripheral blood provides a potential way to detect and characterize cancer. Existing technologies to separate or capture CTCs from whole blood still have issues with sample throughput, separation efficiency or stable efficiency at different flow rates. Here, we proposed a new concept to capture rare CTCs from blood by integrating a triangular prism array-based capture apparatus with streamline-based focus-separation speed reduction design. The focus-separation design could focus and maintain CTCs, while removing a considerable proportion of liquid (98.9%) containing other blood cells to the outlet, therefore, a high CTC capture efficiency could be achieved in the trap arrays with a high initial flow rate. It is worth mentioning that the new design works well over a wide range of flow rates, so it does not require the stability of the flow rate. The results showed that this novel integrated chip can achieve a sample throughput from 5 to 40 mL h−1 with a stable and high CTC capture efficiency (up to 94.8%) and high purity (up to 4 log white blood cells/WBC depletion). The clinical experiment showed that CTCs including CTC clusters were detected in 11/11 (100.0%) patients (mean = 31 CTCs mL−1, median = 25 CTCs mL−1). In summary, our chip enriches and captures CTCs based on physical properties, and it is simple, cheap, fast, and efficient and has low requirements on flow rate, which is very suitable for large-scale application of CTC testing in clinics.


 Please wait while we load your content...
Please wait while we load your content...