Time-resolved microwell cell-pairing array reveals multiple T cell activation profiles†
Abstract
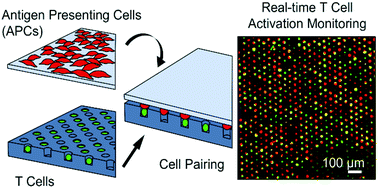
The differences in behaviour between individual cells in a large population are often important, yet are masked in bulk analyses where only average parameters are measured. One unresolved question in the field of immunology is the extent to which important immunological phenomena such as immunodominance to cancer antigens correlates with the average activity of a population of antigen-specific T lymphocytes, or with the activity of individual “outlier” cells. Despite progress in single cell technologies, few platforms are available that can deliver time-resolved, functional analysis at single cell resolution, for these investigations. We have developed an accessible high-throughput platform to measure single T cell signalling in real time following time-controlled stimulation by live antigen presenting cells. The cell-trap array consists of thousands of individual microwells cast in an agarose block, which is biocompatible and permeable to nutrients. Single T cells are isolated in wells via passive sedimentation and size exclusion, achieving up to 90% occupancy. The device enables simultaneous activation of thousands of single CD8+ cells. Stimulation with soluble reagents (ionomycin, anti-CD3 antibodies) or antigen presenting cells leads to changes in intracellular calcium concentrations which were measured using calcium-chelating fluorophore dyes. The platform was used to demonstrate a range of activation profiles among individual cells of a cloned, antigen specific CD8+ T cell hybridoma in response to both nonspecific stimuli and specific, physiologically relevant antigen stimulation. The presence of two different activation profiles was demonstrated, together with rare outlier behaviour among cells that are essentially clonal.


 Please wait while we load your content...
Please wait while we load your content...