Speeding up biphasic reactions with surface nanodroplets†
Abstract
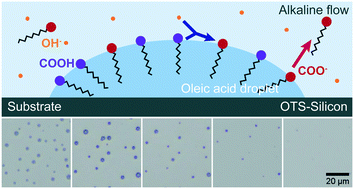
Biphasic chemical reactions compartmentalized in small droplets offer advantages, such as streamlined procedures for chemical analysis, enhanced chemical reaction efficiency and high specificity of conversion. In this work, we experimentally and theoretically investigate the rate for biphasic chemical reactions between acidic nanodroplets on a substrate surface and basic reactants in a surrounding bulk flow. The reaction rate is measured by droplet shrinkage as the product is removed from the droplets by the flow. In our experiments, we determine the dependence of the reaction rate on the flow rate and the solution concentration. The theoretical analysis predicts that the life time τ of the droplets scales with Peclet number Pe and the reactant concentration in the bulk flow cre,bulk as τ ∝ Pe−3/2cre,bulk−1, in good agreement with our experimental results. Furthermore, we found that the product from the reaction on an upstream surface can postpone the droplet reaction on a downstream surface, possibly due to the adsorption of interface-active products on the droplets in the downstream. The time of the delay decreases with increasing Pe of the flow and also with increasing reactant concentration in the flow, following the scaling same as that of the reaction rate with these two parameters. Our findings provide insight for the ultimate aim to enhance droplet reactions under flow conditions.


 Please wait while we load your content...
Please wait while we load your content...