Cell adhesion and proliferation on common 3D printing materials used in stereolithography of microfluidic devices
Abstract
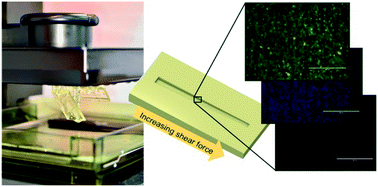
Three-dimensional (3D) printing has recently emerged as a cost-effective alternative for rapid prototyping of microfluidic devices. The feature resolution of stereolithography-based 3D printing is particularly well suited for manufacturing of continuous flow cell culture platforms. Poor cell adhesion or material-induced cell death may, however, limit the introduction of new materials to microfluidic cell culture. In this work, we characterized four commercially available materials commonly used in stereolithography-based 3D printing with respect to long-term (2 month) cell survival on native 3D printed surfaces. Cell proliferation rates, along with material-induced effects on apoptosis and cell survival, were examined in mouse embryonic fibroblasts. Additionally, the feasibility of Dental SG (material with the most favored properties) for culturing of human hepatocytes and human-induced pluripotent stem cells was evaluated. The strength of cell adhesion to Dental SG was further examined over a shear force gradient of 1–89 dyne per cm2 by using a custom-designed microfluidic shear force assay incorporating a 3D printed, tilted and tapered microchannel sealed with a polydimethylsiloxane lid. According to our results, autoclavation of the devices prior to cell seeding played the most important role in facilitating long-term cell survival on the native 3D printed surfaces with the shear force threshold in the range of 3–8 dyne per cm2.


 Please wait while we load your content...
Please wait while we load your content...